SYMPOSIUM PAGE
Register to be among the first Healthcare Professionals to get access to the Symposium Recording and Assets
26th WFHSS World Sterilization Congress,
1 Airport Expo Blvd, Chek Lap Kok, Hong Kong
General Announcement
“Pioneering Excellence in Endoscope Reprocessing”
Despite comprehensive guidelines and technology advances, improper reprocessing of complex reusable devices remains a well-documented cause of device-related infections and outbreaks.1,6-8,12,13
In recent years, new challenges have been associated with innovative surgical techniques, such as robotic and minimally invasive surgery, as well with modifications of endoscopic devices. 2,3
Endoscopy is increasingly used as an alternative form of diagnosis and increasingly invasive treatment, which points to the need to reclassify endoscopes in the Spaulding classification.12,13
Several issues related to incomplete MD reprocessing have been identified that require process improvement in this area.14-17
The reprocessing of the devices used in these procedures is challenging and requires appropriate techniques. Complex devices coupled with instances of inappropriate reprocessing practices have resulted in several outbreaks of infections; some were caused by multidrug-resistant bacteria related to endoscopic procedures, which highlights the importance of adequate reprocessing of the MD used in these techniques. 2,3
Improving the reprocessing of medical devices, particularly endoscopes, should be considered a major target for healthcare institutions to achieve in compliance with the highest standards of quality, efficacy, and safety. 6,7,8
At the same time, the application of the Circular Economy in the healthcare sector, which can be defined as a systems solution framework that tackles global challenges like climate change, biodiversity loss, waste, and pollution, must be regarded as an important framework to foster a better planet and an alternative way to attain economic growth and profits.9
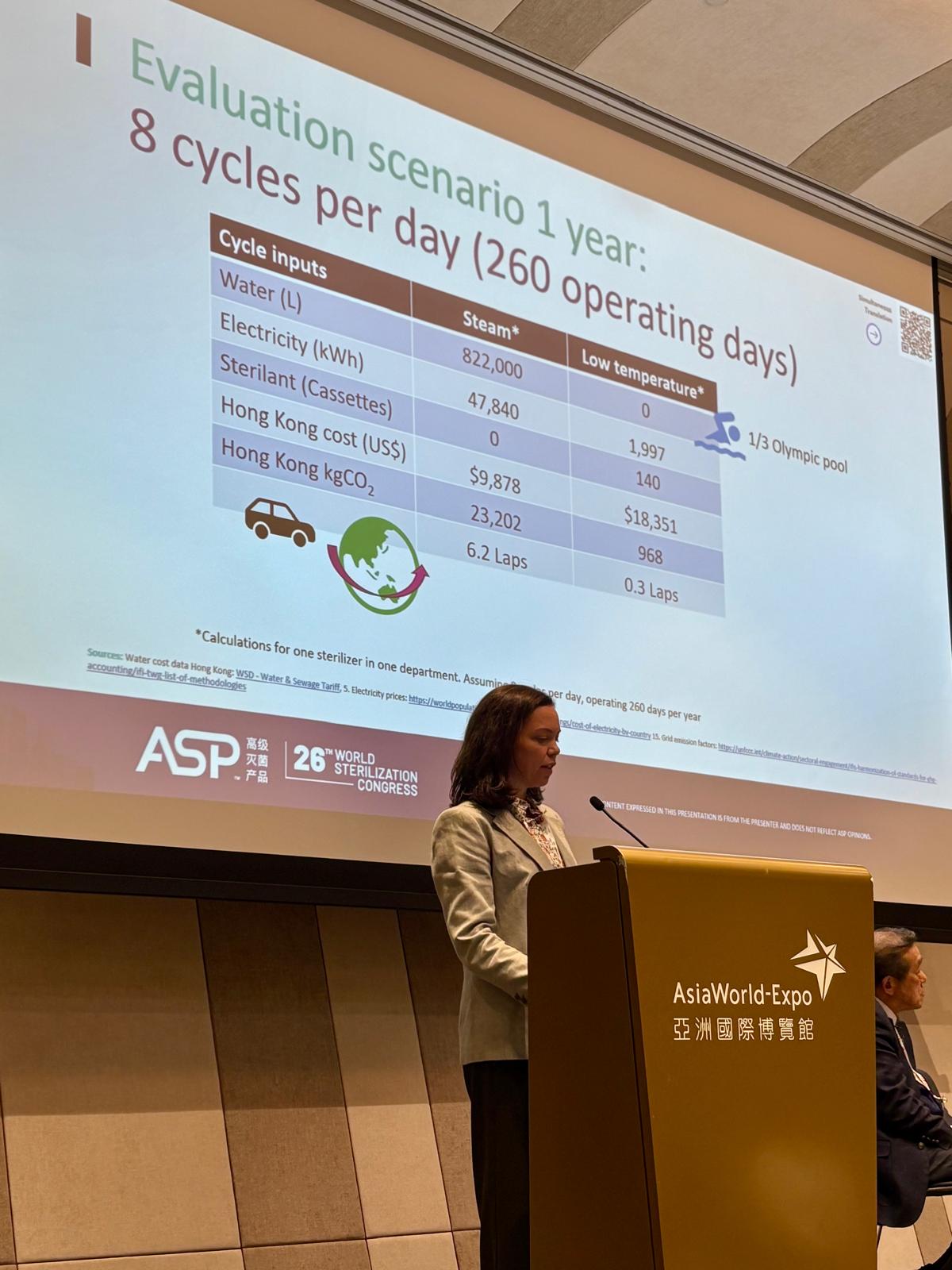
Reprocessing technologies must also take into account economy and sustainability principles in order to support better decision-making. For example, efficient sterilization methods with reduced water and energy consumption mean cost savings and less damage to equipment and the environment, contributing to sustainable healthcare systems and a greener environment.4,5,18-20
New design strategies can be adopted to implement the Circular Economy in the healthcare sector, which depend on the value and the critical degree of the medical devices, resulting in lesser waste and longer durability of the equipment.10 Alternative reprocessing methods, namely low temperature instead of steam, could improve efficiency and result in better economic outcomes for a facility.11
SPEAKERS


Dr. Seto Wing Hong, MD is presently the Co-Director of the WHO Collaborating Center, and Senior Lecturer at the School of Public Health, the University of Hong Kong.
Currently Co-Director, WHO Collaborating Centre for Infectious Disease Epidemiology and Control
Chief Infection Control Officer, Hospital Authority of Hong Kong overseeing the Disinfection and Sterilization guidelines in Hong Kong before retirement.
Received 15 awards in Medical field and 4 National/International medals.
Editorial Board membership for 5 International Peer-Reviewed journals.
Close to 200 publications in reference journal.


Mrs. Patricia Ching is presently the of the WHO Collaborating Center, School of Public Health, the University of Hong Kong.
Post graduate qualifications including Midwifery, Critical Care, Coronary care, Infection Control and epidemiology, Diploma of Nursing Administration.
Comprehensive infection control experience from acute care to long term care and extending to Traditional Chinese Medicine Clinics.
The Hong Kong Academy of Nursing conferred her the Honorary Fellow Member in Infection Prevention and Control in May 2018.
Published widely in infection control and nursing.
Membership: Vice President of APSIC from 2024 – 2026.

Hunter Medical Research Institute

Leads the Health Economics team at Hunter Medical Research Institute (Newcastle, Australia), providing expert advice for competitive research grants and industry consultancy projects.
Specializes in economic evaluations, incorporating environmental footprint alongside costs and health outcomes.
Collaborates nationally and internationally with clinicians and industry to design feasible, value-focused studies aligned with policy and decision-making priorities.
Committed to advancing health economics methods that integrate sustainability and real-world impact.

Advanced Sterilization Products

Vice President and Chief Medical & Scientific Officer at Advanced Sterilization Products (ASP), focused on reducing Hospital Associated Infections and improving standards of care.
Holds an M.D. in Perioperative Medicine and Surgical Critical Care from the Mount Sinai School of Medicine.
Earned B.S. and M.S. degrees in Chemical Engineering from Columbia University, and an M.B.A. from the MIT Sloan School of Management.
Former faculty member at the University of Pennsylvania School of Medicine with 10 years of clinical critical care experience.
Author of 76 peer-reviewed publications and recipient of multiple research grants, including NIH-funded surgical research.

Here are the symposium’s assets
You can download the ebook, watch the symposium’s recording, or listen to the posdcast. Register for free to access.
- Global guidelines for the prevention of surgical site infection, second edition. Geneva: World Health Organization; 2018. (pag.27 3.1 Surgical site infection risk factors: epidemiology and burden worldwide). https://www.who.int/gpsc/ssi-prevention-guidelines/en/
- Ofstead, C. L., Buro, B. L., Hopkins, K. M., Eiland, J. E., Wetzler, H. P., & Lichtenstein, D. R. (2020). Duodenoscope-associated infection prevention: A call for evidence-based decision making. Endoscopy international open, 8(12), E1769–E1781. https://doi.org/10.1055/a-1264-7173
- Scoping the problem – endoscopy associated infections – The Lancet Gastroenterology & Hepatology (www.thelancet.com/gastrohep Vol 3 July 2018)
- Pichler P, Jaccard IS, Weisz U, Weisz H. International comparison of health care carbon footprints. Environ Res Lett. 14(6):064004. 7.
- Health Care Without Harm, Arup. Health care’s climate footprint. Health Care Without Harm; 2019. https://noharm-global.org/documents/health-care-climate-footprint-report.
- Dancer, S. J., Stewart, M., Coulombe, C., Gregori, A., & Virdi, M. (2012). Surgical site infections linked to contaminated surgical instruments. The Journal of hospital infection, 81(4), 231–238. https://doi.org/10.1016/j.jhin.2012.04.023. Journal Hosp Infection 2012 Aug;81(4):231-8. doi: 10.1016/j.jhin.2012.04.023. Epub 2012 Jun 15.
- Southworth P. M. (2014). Infections and exposures: reported incidents associated with unsuccessful decontamination of reusable surgical instruments. The Journal of hospital infection, 88(3), 127–131. https://doi.org/10.1016/j.jhin.2014.08.007
- Tosh, P. K., Disbot, M., Duffy, J. M., Boom, M. L., Heseltine, G., Srinivasan, A., Gould, C. V., & Berríos[1]Torres, S. I. (2011). Outbreak of Pseudomonas aeruginosa surgical site infections after arthroscopic procedures: Texas, 2009. Infection control and hospital epidemiology, 32(12), 1179–1186. https://doi.org/10.1086/662712
- https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview
- G.M. Kane, C.A. Bakker, A.R. Balkenende. Towards design strategies for circular medical products. Resources, Conservation and Recycling, Volume 135, 2018, Pages 38-47, https://doi.org/10.1016/j.resconrec.2017.07.030)
- McCreanor, V., & Graves, N. (2017). An economic analysis of the benefits of sterilizing medical instruments in low-temperature systems instead of steam. American journal of infection control, 45(7), 756–760. https://doi.org/10.1016/j.ajic.2017.02.026
- Rutala, W., Weber, D.. 2013. New Developments in Reprocessing Semicritical Items. American Journal of Infection Control. Elsevier Inc. pp. 560-566. https://doi.org/10.1016/j.ajic.2012.09.028.
- Rational Approach _ Disinfection & Sterilization Guidelines_ CDC https://www.cdc.gov/infectioncontrol/guidelines/disinfection/rational-approach.html.
- Pyrek, K. M. August 10, 2018. Device-Related Infections: Patient-Ready Bronchoscopes Found to be Contaminated Despite Cleaning and Disinfection. Infection Control Today. https://www.infectioncontroltoday.com/view/devicerelated-infections-patient-ready-bronchoscopesfound-be-contaminated;
- Ofstead, C. L., Heymann, O. L., Quick, M. R., Johnson, E. A., Eiland, J. E., & Wetzler, H. P. (2017). The effectiveness of sterilization for flexible ureteroscopes: A real-world study. American journal of infection control, 45(8), 888–895. https://doi.org/10.1016/j.ajic.2017.03.016.
- Center for Devices and Radiological Health. (2022d). UPDATE: Change in reprocessing methods with certain Karl Storz Urological endoscopes – Letter to health care providers. U.S. Food and Drug Administration. https://www.fda.gov/medical-devices/letters-health-careproviders/update-change-reprocessing-methods-certain-karl-storz-urological-endoscopes-letter-health-care.
- Food and Drug Administration (FDA). September 17, 2015. Infections Associated with Reprocessed Flexible Bronchoscopes: FDA Safety Communication. Retrieved June 8, 2023 from https://www.fdanews.com/ext/resources/files/09- 15/092115-safety-notice.pdf?1520852028
- McCreanor, V., & Graves, N. (2017). An economic analysis of the benefits of sterilizing medical instruments in low-temperature systems instead of steam. American journal of infection control, 45(7), 756–760. https://doi.org/10.1016/j.ajic.2017.02.026.
- Schafer B. Decreased number of repairs of rigid scopes as a result of low-temperature sterilization with H2O2 gas plasma. A field report form the Barmherzige Bruder Hospital in Trier, Germany. Central Serv 2009;17:194-6.
- Skogas J, Marvik R. Measures taken to reduce damage and repair costs of rigid endoscopes during their handling and processing in surgical practice. Minim Invasive Ther Allied Technol 2003;12:76-81.