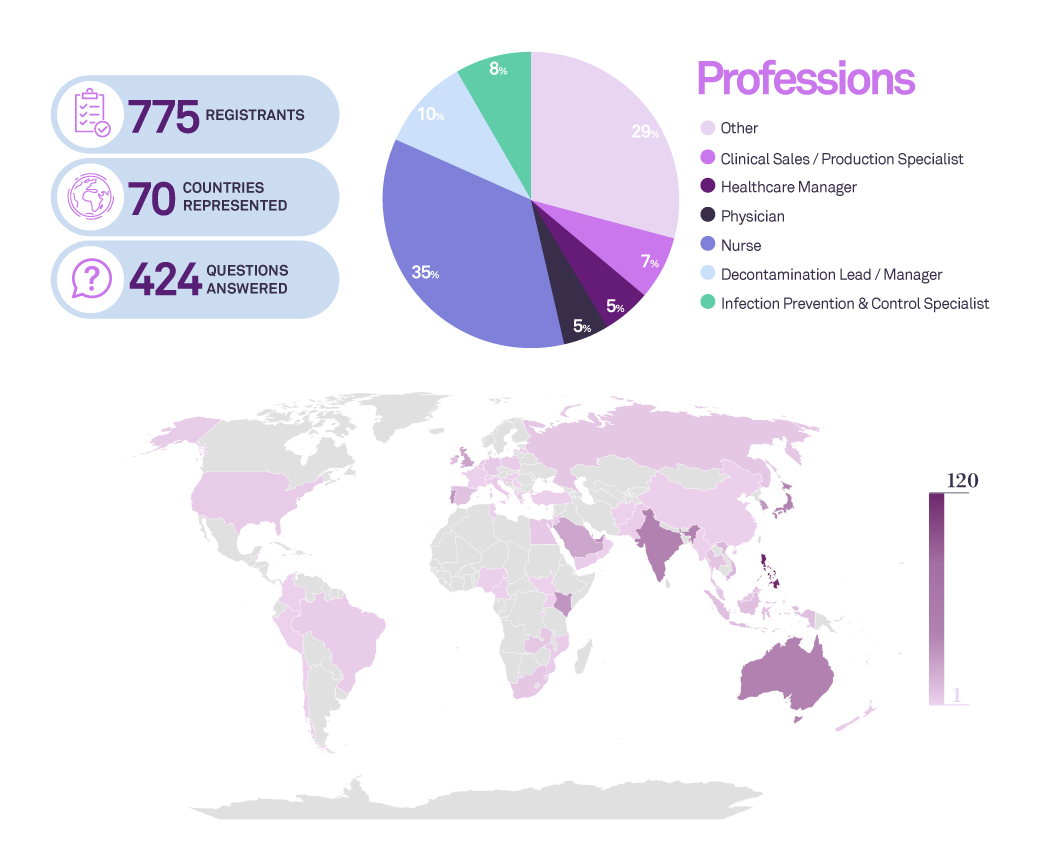
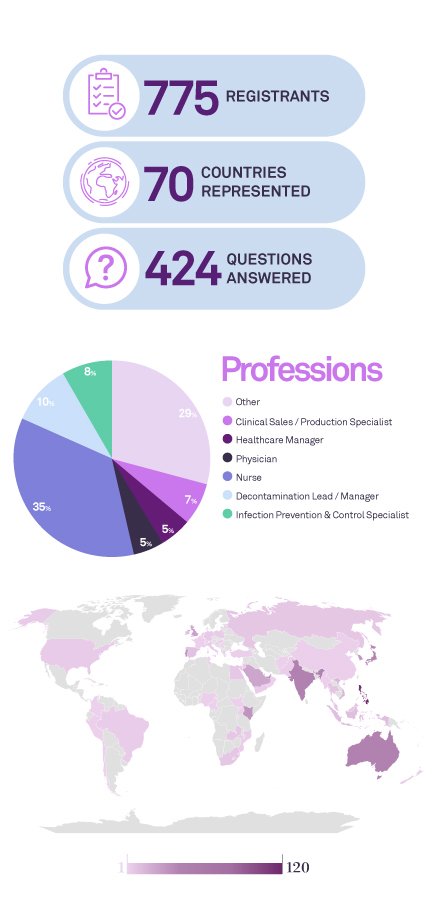
Endoscope reprocessing is a challenge for healthcare professionals and institutions. In the past years there has been several references to outbreaks which the root cause was related with limitations of the endoscope decontamination. In this webinar we will discuss the current gaps in flexible endoscope decontamination, the inadequacy of sampling and test methods for biofilm, the risk of biofilm, the potential of biofilm accumulation in flexible endoscopes, and what can or should we do to combat this.
We start with an overview of the 3 areas (decontamination processes, testing and what are biofilms) we then look to what can we do to improve these processes to give greater assurance to our patients, the main take home point is that current practice is not enough, sterilisation should be adopted and we look at real world studies showing why gas plasma is the practical solution to the challenges presented.
In this topic, we will discuss the current processes as per standards and guidance for endoscope decontamination. We then link this to Spaulding with a view that scope technology has advanced but endoscopes still remain on the original 1957 classification. Therefore, we ask: should these be classified as critical due to their application in modern times.
In this topic, we will review the assurance test methods adopted by standards looking at what is done to give assurance of machine performance and water quality but then focus on the small amount of assurance tests by comparison that are conducted on the actual devices.
In this section, we will review the Biofilms, their risk and the potential accumulation in endoscopes. We start by explaining what biofilms are, how they present a challenge to decontamination and the limited tests available to identify their presence. This is where we will look at some recent studies demonstrating the risk of residual contamination on endoscopes following full processing.
In this section we will discuss the future of endoscope reprocessing to ensure safety of patient ready devices. We will also bring together the themes from the previous topics and present the audience with suggestions for risk assessing patients, the need to sterilise scopes and the efficacy of Gas Plasma being the solution.
This is the third webinar dedicated to “Flexible Endoscope Decontamination: The Challenge of Biofilms!”, presented by ASP Continuous Education.
17th September 2025

Trust Decontamination Lead
IDSc Director of Communication & Marketing
Chairman IDSc Midlands Branch
- Trust Decontamination Lead for Manchester University Hospitals NHS Foundation Trust, the largest Healthcare provider in Northern Europe, 10 Acute care hospitals, 7 community medical centers. 30,000 staff and over £2.6 billion turnover.
- A Director & Regional Chairman for the Institute of Decontamination Sciences the UKs largest professional body for Decontamination.
- Registered Healthcare Scientist specialising in Decontamination and Infection Prevention and Control.
- Published in 2012 for research conducted with Public Health England, looking at cleaning efficacy of chemistries on TSE (CJD) Prions.
- Over 16 years’ experience in Decontamination Science, being one of the first in the UK to centralise Endoscope decontamination and have processes accredited to ISO13485 and MDR back in 2009.
- Currently conducting extensive research into biofilms with a view to challenge international standards and guidance documents, particularly for Flexible Endoscope Decontamination.